coursework
Production and industrial technologies
Deoxidizing capacity and other properties of deoxidizing elements The most important deoxidizing agents Study various ways steel deoxidation Precipitating deoxidation Extractive diffusion deoxidation Vacuum carbon deoxidation...
43
INTRODUCTION
1. ANALYTICAL PART
-
- The main tasks of deoxidation and requirements for deoxidizing elements
- Deoxidizing capacity and other properties of deoxidizing elements
- The most important deoxidizers
- The study of various methods of steel deoxidation
- Precipitating deoxidation
- Extraction (diffusion) deoxidation
- Vacuum carbon deoxidation
- Deoxidation in a steelmaking plant
- Deoxidation in a steel ladle
- Deoxidation in the mold
-
- Goals and objectives of preliminary deoxidation
- STATEMENT OF OBJECTIVES IN WORK
- CONCLUSIONS ON COURSE WORK
INTRODUCTION
The ever-growing requirements for the quality of finished steel products, and the ever-increasing prices for raw materials, equipment for the metallurgical complex, force technologists to develop more and more new ways to improve product quality with minimal cost. The operation of reducing the residual oxygen content in the metal after the oxidation period of melting (deoxidation) is no exception, without which not a single steelmaking process can do today. The oxidizing conditions of melting in steelmaking units, the presence of oxidizing slags, as well as the interaction of the metal with the atmosphere during tapping and pouring - all this is a prerequisite for the fact that oxygen dissolved in steel has a certain and often increased activity by the time it is tapped from the unit. The technological operation that reduces the activity of oxygen to the required limits is called deoxidation. Steel that has undergone such treatment is called deoxidized. If the deoxidized steel behaves calmly during solidification in the molds, i.e., almost no gases are emitted from it, then such steel is called calm. If deoxidation is not carried out, then in steel with its gradual cooling in the mold, a reaction will occur between dissolved oxygen and metal carbon:
The bubbles of carbon monoxide formed in this case, escaping from the crystallizing ingot, lead to the fact that the metal in the mold is intensively mixed, its surface boils. Such steel is called boiling steel. Sometimes not all oxygen is removed from steel during deoxidation. The remaining dissolved oxygen causes the metal to boil for a short time (40 seconds). Such steel is called semi-calm.
Deoxidation is a mandatory operation in the smelting of steel of any grade and , being one of the final operations for obtaining a given content of impurities in the finished steel, to a large extent determines the quality of the ingot and finished products from it, so this operation is very responsible and requires special attention.
1. ANALYTICAL PART
- Analysis of the steel deoxidation process
- The main tasks of deoxidation and requirements for deoxidizing elements
Numerous studies of the patterns of changes in the oxygen content in the metal at the end of the oxidative refining process (before deoxidation) made it possible to conclude (Fig. 1.2) that the oxygen content in the metal before deoxidation in any steelmaking plant mainly depends on the carbon concentration: the lower the carbon content, the more oxygen content in the metal. This oxygen concentration is much higher than the equilibrium concentration with carbon. If this oxygen content is preserved in the metal, then during the solidification of steel in the mold of a continuous casting machine (CCM) in the mold or mold, the carbon oxidation reaction and gas evolution will continue. This is permissible only if boiling and semi-quiet steels are smelted, and the intensity of gas evolution in the mold must be quite certain: when the boiling metal solidifies more (but not excessively), athardening semi-calm less. During solidification of the ingot of calm steel, visible gas evolution, i.e. the course of the carbon oxidation reaction must be excluded.
The level of activity (concentration) of oxygen achieved during deoxidation is called the degree of deoxidation. The structure of the steel ingot depends on the degree of deoxidation (Fig. 1.1).
On fig. 1.1 shows curves characterizing the level of oxidation of steel after its deoxidation. According to this scheme, the deoxidation of boiling steel is reduced only to a certain decrease in the oxygen content in the metal (the level of oxidation of the metal remains above the equilibrium level with carbon). Most often, this is ensured by the introduction of a certain amount of manganese (sometimes small amounts of silicon and aluminum are also introduced into the metal). Usually boiling steel contains
Figure 1.1 - Schematic structures of steel ingots:
1 - calm; 2 - semi-calm; 3 - clogged; 4.5 - boiling metals. The numbers near the lines are the concentration of oxygen in steel
Consequently, the first task of steel deoxidation is reduced to achieving a given degree of metal deoxidation - obtaining in the finished liquid steel such a residual oxygen content that ensures the normal behavior of the metal during its crystallization. The meaning of solving this problem is illustrated by the diagram in Fig. 1.2
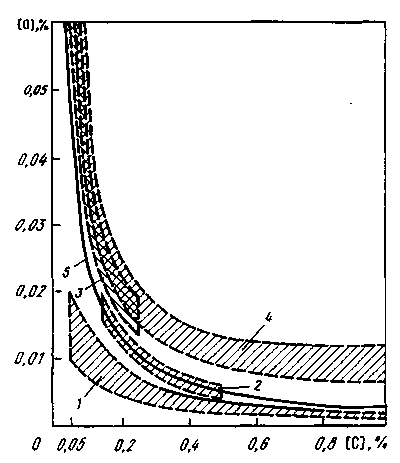
Figure 1.2 - The level of oxidation of steel after its deoxidation:
1, 2, 3 - in the production of calm, semi-quiet and boiling steel, respectively; 4 – area of normal oxygen content in the metal before deoxidation; 5 - equilibrium curve with carbon
The deoxidation of boiling steel is reduced only to a certain decrease in the oxygen content in the metal while maintaining its level above the equilibrium level with carbon. This is usually ensured by deoxidation only of manganese with a residual content of 0.3–0.4%; silicon is rarely additionally introduced (the residual content is not more than 0.02–0.03%) and aluminum (thousandths of a percent).
The deoxidation of semi-dead steel means obtaining a residual oxygen content in the metal slightly below equilibrium, usually. Only when this condition is met, an ingot of semi-quiet steel is formed normally: the carbon oxidation reaction proceeds only to the extent that it is necessary to fill the shrinkage voids inevitably formed during steel crystallization with gases. Obtaining such a residual oxygen content is not an easy task, since a slight over or under deoxidation leads to a disruption in the normal course of crystallization of the ingot. In most cases, when deoxidizing semi-smooth steel, in addition to the usual manganese content, it is sufficient to have residual silicon in the finished steel in the final metal or a few thousandths of a percent of aluminum.
The deoxidation of calm steel can be considered normal if the residual oxygen content is significantly lower than the equilibrium content with carbon. In this case, the lower the residual oxygen content, the better, therefore, the deoxidation of calm steel is practically reduced to the introduction of one or more deoxidizing elements into the metal, which have a high chemical affinity for oxygen. For example, in most cases it is sufficient to have a residual content in the finished metal. However, when smelting steel, especially calm steel, the task of deoxidation is not limited to obtaining the required oxygen content in the metal.
The second task of deoxidation is to ensure the lowest possible content in solid steel of the products of deoxidation reactions - non-metallic inclusions (NI), as well as to obtain NI that have a minimal negative effect on the properties of steel. Such properties are possessed by small HB (), having the shape of a sphere, located in the volume of the metal evenly and not deformed during pressure treatment. This problem is very complex and has been successfully solved so far only in a few cases.
The third task of deoxidation is reduced to ensuring the production of a fine-grained structure of the metal and is solved by obtaining fine NIs that stand out from liquid steel in solid form and play the role of centers for the formation of metal crystals. Nitrides and carbontrides of vanadium, niobium, etc. have such properties. In this case, HBs have a positive effect on the properties of steel.
In most cases, a deoxidizing element is introduced into the metal not only to reduce the residual oxygen content, but also to reduce the harmful effects of other impurities, as well as to improve the properties of steel (heat machinability, mechanical strength, corrosion resistance, etc.). Fulfillment of these requirements is possible, as a rule, only at certain contents of deoxidizing elements in the metal, the limits of which are set during the development of steel of this grade, therefore, for the technologist conducting melting, in the end, the task of deoxidation-alloying is reduced to obtaining a given content of deoxidizing agents in the finished steel. and alloying elements.
As is clear from the above, deoxidizing elements must have the following properties:
1) high breaking capacity (high chemical affinity for oxygen);
2) a tendency to form oxides that are insoluble in liquid steel, easily removed from it or causing minimal damage to its properties;
3) the ability to improve the properties of steel (increase in strength, heat machinability, resistance to aggressive environments etc.);
4) low cost and availability. In addition, the deoxidizing element should help reduce the negative effect on the properties of steel of other harmful impurities, except for oxygen: sulfur and nitrogen, and the deoxidation products, remaining in the steel, should contribute to grain refinement.
1.1.2 Deoxidizing capacity and other properties of deoxidizing elements
The deoxidizing ability of deoxidizing elements, their most important characteristic, is usually estimated by the residual equilibrium oxygen concentration in the metal corresponding to the given deoxidizer content and the accepted temperature: the lower the residual oxygen content, the higher the deoxidizing ability of the element.
The deoxidizing capacity can be determined experimentally or by calculation, based on the equilibrium conditions of the deoxidation reaction, which can be generally represented by the expression: where: is the residual content of the deoxidizing element in the metal; - the product of the deoxidation reaction, which can be a pure solid oxide of a certain chemical composition ( m and n are stoichiometric coefficients), an alloy or a chemical compound of the formed oxide with or oxides of other elements (silicates, aluminates, rnnlides, etc.). These alloys and compounds, as a rule, have a variable composition, i.e. in this case, m and n are variable values and may not reflect the stoichiometric ratios characteristic of a particular oxide. The equilibrium constant of the deoxidation reaction is given by the expression
Where: are the activities of the oxide, oxygen and deoxidizing element, respectively; – equilibrium residual concentrations of oxygen and deoxidizing element after deoxidation, %; are the activity coefficients of oxygen and the deoxidizing element.
From the expression of the equilibrium constant we find:
It follows from the equation that the equilibrium residual oxygen content in the metal after deoxidation depends on a large number of factors, the lower the activity of the resulting deoxidation product, the greater the equilibrium constant, the residual concentration of the deoxidizing element in the metal and the activity coefficient of it and oxygen, and each of these factors can vary within certain limits, causing a corresponding change in the degree (depth) of deoxidation.
The residual content of the deoxidizing element is an important factor, which determines the degree of deoxidation, and it is always more or less certain, since the deoxidation process can be carried out in such a way that the content of the deoxidizer in the final metal e is within the specified limits.
The activity coefficient of the deoxidizing element usually differs slightly from unity, since the concentration of the deoxidizing agent in the metal is usually low. The activity coefficient of the deoxidizing element is taken equal to 1.
The equilibrium constant in the general case for different elements is a variable factor, since it characterizes the chemical affinity of elements for oxygen, which can vary by several orders of magnitude. The equilibrium constant for a given element depends on temperature. Due to the exothermicity of all deoxidation reactions, the value decreases with increasing temperature, which causes an increase, i.e. reduction in deoxidizing capacity. Due to the temperature change of the metal at the end of the process within narrow limits, the effect of temperature change is usually neglected (the deoxidation temperature is assumed to be constant, equal to 1600°C).
The activity of the deoxidation product depends on the form in which it is released. It can be taken equal to 1 if the oxide is isolated in its pure form. In the case of the transition of the resulting oxide into the finished slag (slag inclusion) or its interaction with other oxides of the activity of the deoxidation product, therefore, the deoxidizing ability of the element is higher. Numerous studies have established that deoxidation products can be different even for the same element. In this case, the following regularity is observed: in the region of low concentrations of the element, the deoxidation product usually represents a compound (and others) or a melt of these oxides (), i.e. richer in oxygen than the pure oxide of the deoxidizing element. In the area of high concentrations of the element, the deoxidation product can be pure oxide (if it is released from liquid steel in solid form and there are no other non-metallic inclusions in the metal that can interact with them - to form a chemical compound or melt. When the deoxidation product is released in liquid form, the content in it decreases gradually as the concentration of the element in the metal decreases.
It is characteristic that the higher the chemical affinity of the element for oxygen, the lower its concentration in the metal is observed the release of the deoxidation product in the form of pure oxide. For example, in the case of deoxidation with titanium, pure oxide or is released with a residual content of titanium and, according to Chipman, a much lower residual content of aluminum is required to isolate pure oxide. When deoxidized only by manganese, the formation of a melt of variable composition is observed. If deoxidation is carried out simultaneously with manganese and silicon, then a silicate is formed, as a result of which the deoxidizing ability of both manganese and silicon increases. Thus, the activity of the deoxidation product can generally vary within wide limits, and it is difficult to control this change. Only in the particular case, when a strong deoxidizer is used, can it be considered constant, equal to 1.
The oxygen activity coefficient in the general case can also vary over a wide range, since it is affected by the content of both oxygen and impurities dissolved in the metal, i.e.
where: is the oxygen activity coefficient in a real bath, when the metal contains several deoxidizing elements.
In such complex systems, the oxygen activity coefficient can be determined from the equation:
where: - interaction parameters characterizing the degree of influence of the concentration of one or another element on the value. All deoxidizers reduce i.e. have:
|
0,03 |
0,14 |
0,27 |
0,36 |
0,94 |
0,4 |
As can be seen from the above data, the greater the chemical affinity of an element for oxygen, the higher the interaction parameter, i.e. strong deoxidizers not only reduce the content of residual oxygen in the metal, but also reduce the activity. Interaction parameter or, i.e. the effect of oxygen concentration on its activity can be neglected. In the case of content of manganese and silicon and; in this case, neglecting the effect of deoxidizing elements on leads to significant errors. It is all the more unacceptable if the deoxidation is carried out with a stronger deoxidizer. For an approximate assessment of the deoxidizing ability of elements, they are usually taken, i.e. It is assumed that the liquid mechall is an ideal dilute solution in terms of the content of oxygen and deoxidizing elements. Close to this is the usual carbon steel. In addition, they accept, i.e. allow the formation of pure oxide, which is possible when deoxidized with one strong (non-complex) deoxidizer. Then:
the value conventionally called the equilibrium constant. Having experimentally determined the equilibrium relations for simple systems at different temperatures, find temperature dependence:
For a given element and a given temperature Ke, the value is constant; therefore, i.e. the deoxidizing power depends only on the concentration of the element in the metal. On fig. 1.3 shows data on the deoxidizing capacity of the main deoxidizing elements obtained as a result of these simplifications. Since the deoxidizing ability of the elements differs by several orders of magnitude, the diagram in Fig. 1.3 has logarithmic scales, i.e. for its construction, the following equation was adopted:
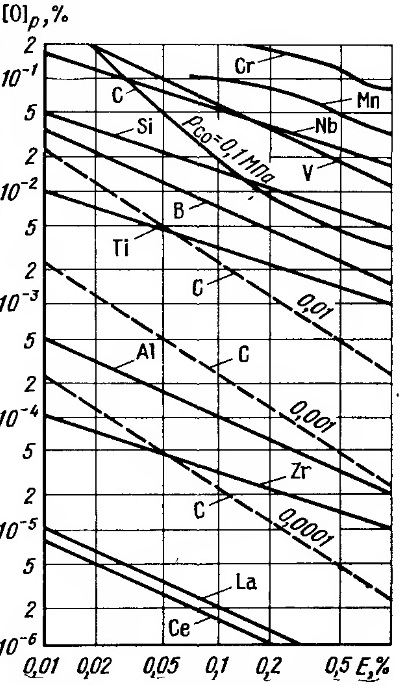
Figure 1.3 - Deoxidizing ability of elements at 1600 (– equilibrium oxygen content)
Such a construction of the diagram is also convenient because the tangent of the slope of the straight lines on it expresses the ratio m /i, i.e. allows you to set the possible ratio of the deoxidizer and oxygen in the deoxidation product (to determine its chemical composition). The composition of the deoxidation product changes with the concentration of the deoxidizer. Therefore, in the diagram in Fig. 1.3 the line characterizing the deoxidizing ability of an element cannot be straight, it must be a curve, similar to the lines for manganese and chromium. On fig. 1.3 also does not reflect a possible increase in the concentration of residual oxygen with an increase in the content of the deoxidizer above certain limits (for chromium - titanium 0.8%, aluminum 0.2%, etc.). Diagram fig. 1.3, although simplified, allows an approximate estimate of the deoxidizing capacity. When constructing its values, they were calculated according to the data in Table. 1.1, which includes the results of studies that are recognized as the most reliable.
Table 1.1
Main deoxidation reactions and their thermodynamic characteristics
|
Reaction |
Temperature dependence of the logarithm of the equilibrium constant |
Oxygen activity at |
Researcher |
|
(-14270/G)+5.70 |
Chipman |
||
|
(-14360/G)+5.92 |
Chipman and Eliot |
||
|
(-14575/G)+5.50 |
Chipman and Gokken |
||
|
(-14897/G)+ 5.14 |
Chino and Wada |
||
|
(-15350/G)+5.17 |
Laulis and Samarin |
||
|
(-21630/G)+6.87 |
Chino and Wada |
||
|
(-20685/G)+6.04 |
Chipman |
||
|
(-20670/G)+4.67 |
Kinne and others. |
||
|
(-25330/G)+7.00 |
With the simultaneous introduction of several deoxidizers, their deoxidizing ability should be higher than the values shown in Fig. 57, since the activity of the resulting oxides. This is the basis for obtaining the so-called complex deoxidizers, which include two or more deoxidizer elements. Such deoxidizers must contain components of two types: a component, the oxidation of which should lead to the formation of a basic oxide, and a component, the oxidation of which gives an acidic or amphoteric oxide.
In addition, when compiling a complex deoxidizer, it is necessary to take into account the possibility of the formation of strong chemical compounds between the components. When such compounds are formed, the activity of deoxidizing elements and the deoxidizing ability of the alloy decrease. Such compounds include silicides of chromium, vanadium, niobium and some other materials. Therefore, if it is necessary to increase the deoxidizing ability of the alloy, silicon cannot be combined with the indicated metals. In other cases, such combinations are possible. For example, in the production of steel, silicochrome is used, in which silicon is a useful component that reduces the melting point of the alloy and accelerates its dissolution in liquid iron. With the correct composition of the complex deoxidizer, not only the deoxidizing ability of its components increases, but it can also be ensured that such deoxidation products are obtained that are better removed from the metal or, remaining in the metal, have a minimal negative effect on its quality.
The ability to form stable sulfides is important property deoxidizing elements. However, this property of the elements has been studied much less than their deoxidizing ability. Available production and laboratory data indicate that the chemical affinity for sulfur elements are arranged in a different sequence than the chemical affinity for oxygen. So, silicon, which has a significant ability to form oxides, practically does not participate in the formation of sulfides, and manganese is a good sulfide-forming element, although it is much weaker than silicon in chemical affinity for oxygen. The strongest sulfide-forming elements, in order of increasing their chemical affinity for sulfur, can be arranged in a row: aluminum, alkaline earth metal (), REM (). Currently, the high chemical affinity of AEM and REM for sulfur is used for deep desulfurization of steel in a ladle (obtaining).
The ability to form nitrides is also a valuable property of deoxidizing elements. In descending order of chemical affinity for nitrogen, deoxidizers can be arranged in a row: . The location of the elements in the given series is approximate, since the chemical affinity is estimated from the value of the standard thermal effect of the formation of the corresponding nitrides. Other, more reliable thermodynamic data for most reactions of nitride formation in liquid iron are not available.
The ability to regulate (grind) the primary grain in cases of steel smelting subjected to heat treatment, is one of the important requirements for deoxidizing elements, since the susceptibility of steel to heat treatment depends on the size of the austenite grain: the finer the grain, the better the heat workability, therefore, the higher the quality of the steel (after quenching). In addition, the fine-grained structure of the metal makes it possible to sharply reduce the anisotropy of the mechanical, primarily strength, properties of rolled products in the longitudinal and transverse directions. In a number of cases, for example, in pipes of gas and oil pipelines, this is of paramount importance, therefore, at present, the production of fine-grained steel is carried out in large volumes, and mainly with vanadium alloying.
The size of the primary grain depends on the nature, size and distribution of hard non-metallic submicroscopic particles contained in liquid steel. These particles are mainly formed as a result of the interaction of deoxidizing elements with nitrogen and carbon. The most common element introduced into the metal for grain refinement is vanadium, since it forms both nitrides and carbides. To improve the quality of heat-treated steel, it is important not only to obtain fine grain, but also its stability. The most stable grain is obtained by deoxidation with titanium. Next, in order of decreasing grain stability, are steels deoxidized.
1.1.3 The most important deoxidizers
Currently, there is not a single deoxidizer that would be the best in all the requirements. Some deoxidizers do not have a universal effect on the properties of steel, others, being more or less universal, turn out to be scarce and expensive, therefore, a relatively large number of deoxidizers are used in industrial practice, each of which turns out to be more or less suitable for certain cases.
Manganese is the most common deoxidizer. Manganese-containing alloy - ferromanganese () - is cheap and relatively affordable (at least in Ukraine). The deoxidizing ability of manganese is sufficient to obtain normal ingots of boiling steel. Manganese has a high chemical affinity for sulfur and significantly reduces the negative effect of sulfur on the properties of steel when it is introduced in an amount or higher.
Silicon is also a fairly common deoxidizer. Silicon is introduced into steel in the form of ferrosilicon with low (and high () silicon content. In rare cases, crystalline silicon is used, which (like metallic manganese) is very expensive. The advantage of silicon as a deoxidizer is its high chemical affinity for oxygen, which makes it possible to obtain a calm steel with its residual content in the metal as well as the ability to form nitrides (and prevent steel aging.
Aluminum, which is usually used commercially pure, is one of the best deoxidizers in terms of its physical and chemical properties, since it simultaneously has a high chemical affinity for three harmful impurities - oxygen, nitrogen and sulfur, and also contributes to the refinement of austenite grains. At the same time, the positive effect of aluminum on the properties of steel is felt when its residual content is in hundredths of a percent; therefore, aluminum as a deoxidizer has been increasingly used in recent years, although it is relatively expensive.
Vanadium is a valuable deoxidizer that has a positive effect on the properties of steel in many ways, for example, the production of ageless boiling steel is possible only with the use of vanadium. When introduced into the metal, the residual oxygen content is sufficient for the normal boiling of the metal in the mold, and at this concentration of vanadium, the tendency of steel to age is eliminated. At the same relatively low concentrations, a fine-grained structure and an increase in strength, wear resistance and other service properties of structural, rail, spring and other steels are provided. Vanadium is introduced into steel, usually in the form of ferrovanadium (), which is an expensive and scarce material.
Titanium and zirconium are very good deoxidizers, but ferrotitanium (), ferrozirconium () and other alloys, in the form of which these elements are introduced into steel, are expensive and scarce, therefore, like some other elements (niobium, rare earth metals, etc.) ), usually used only in the production of special-purpose steels.
Calcium and magnesium are the strongest deoxidizers: their use improves the quality of steel. This is explained as follows:
1) their high chemical affinity for oxygen and sulfur, which makes it possible to provide very low residual contents of dissolved oxygen and sulfur in the finished metal;
2) the deoxidation products that remain in the metal form small globular oxysulfide non-metallic inclusions, uniformly distributed in the volume of the metal and slightly deformable during rolling, due to which they have a minimal negative effect on the properties of the steel.
At present, calcium deoxidation, which is part of complex alloys, such as silicocalcium (), ferroaluminosilicocalcium (), etc., has become widespread. It is characteristic that the positive effect of calcium on the properties of steel is already felt when it is consumed and consumption is not required.
Rare earth metals (REM) also have very good deoxidizing properties, they have a high chemical affinity for harmful impurities (oxygen, sulfur and nitrogen). Their melting point is low (), the boiling point is high (), so REM can be introduced into the metal not only in the ladle, but also in the mold. This ensures their normal dissolution and uniform distribution in the metal, evaporation is practically absent. It is advisable to use REM in the form of a complex alloy obtained by a cheap carbon thermal method and containing (mainly cerium) and
Carbon is an ideal deoxidizer, since the CO deoxidation product is removed from the metal. But the high deoxidizing ability of carbon is manifested only when the metal is evacuated and blown with neutral gases, when a low partial pressure of CO in the gas phase is provided. Under normal conditions, the deoxidizing ability of carbon can only be used to deoxidize slag during the extraction (diffusion) deoxidation of the metal.
1.2 Study of various methods of steel deoxidation
According to the principle of removing oxygen from the metal, there are precipitating, extraction (diffusion) and vacuum carbon deoxidation, according to the place of the process - deoxidation in a steel-smelting unit, in a steel-pouring ladle and in a mold.
1.2.1 Precipitating deoxidation
Precipitating deoxidation consists in the fact that the main part of the oxygen dissolved in the metal is converted into insoluble oxides of deoxidizing elements introduced directly into the steel. The formation of insoluble oxides (“precipitate”) determines the name of the deoxidation method. The density of the vast majority of oxides formed is less than the density of liquid steel, so they do not settle, as in aqueous solutions, but float, which leads to their partial removal from the metal into slag.
The tasks of precipitating deoxidation are:
- reducing the solubility of oxygen by additives of deoxidizing elements, characterized by a higher chemical affinity for oxygen than iron, to a level that ensures the production of a dense metal;
- creation of conditions for the possible complete removal of the resulting deoxidation products from liquid steel.
This deoxidation method is often also referred to as "deep" deoxidizers, since the deoxidizers are introduced deep into the metal. Manganese (in the form of ferromanganese), silicon (in the form of ferrosilicon), aluminum, REM alloys (cesium, lanthanum, etc.) and alkaline earth metals are usually used as deoxidizers.
Deoxidation is carried out according to the following reaction:
All these oxidation reactions proceed with the release of heat. The equilibrium of the precipitating deoxidation reaction shifts to the left as the temperature rises and to the right as the temperature falls. In practice, this means that as the temperature of the steel decreases (during its crystallization in the mold or in the casting mold), deoxidation reactions continue to occur and more and more oxides are formed that do not have time to float up and be removed from the metal. In this regard, when this method deoxidation, it is impossible to obtain steel completely free of non-metallic inclusions, which is its disadvantage. However, this method is widely used as the simplest and cheapest.
1.2.2 Extraction (diffusion) deoxidation
Extraction (diffusion) deoxidation is reduced to bringing the metal into contact with slag, which has an oxidation content (iron oxide content) many times lower than the slag of the oxidative refining period. At the same time, in accordance with the distribution law, the oxygen concentration in the metal decreases, tending to equilibrium with the new deoxidizing slag. The minimum possible residual oxygen content after extraction deoxidation can be approximately determined by the following equation, which follows from the expression for the oxygen distribution constant between slag and metal:
where: – residual oxygen content in metal, %; – content of iron oxide in slag, %; is the oxygen distribution coefficient.
As can be seen from the dependence, the degree of extraction deoxidation at a given temperature depends on the content. The minimum content in the slag can be obtained in electric arc furnaces. In open-hearth furnaces, it is difficult to reduce the content up to. At the same time, the bath does not boil for a significant period of time, which leads to saturation of the metal with hydrogen.
During diffusion deoxidation, there is no carbon boiling, since the oxygen content in the metal decreases rapidly and practically reaches equilibrium with the carbon contained in the metal (Fig. 1.4)

Figure 1.4 - Dependence of the oxygen content in the metal on the carbon content before (a) and after (b) diffusion deoxidation
Diffusion deoxidation is carried out using the following types of slag:
- white slag obtained as a result of deoxidation of highly basic slag, first with carbon and then with silicon;
- white slag obtained as a result of deoxidation of highly basic slag only with silicon;
- carbide slag obtained by deoxidation of highly basic slag only with carbon materials with the formation of calcium carbide in the slag
- magnesia-silica and magnesia-alumina slags.
Composition of white slag:
Composition of carbide slag:
Advantages of diffusion deoxidation of steel:
- reduction of oxygen content in steel without the formation of a non-metallic phase in the metal - obtaining pure metal;
- obstruction of the flow of oxygen from the atmosphere of the furnace.
Disadvantages of diffusion deoxidation of steel:
- a significant time-consuming removal of oxygen occurs in the diffusion mode in the absence of natural mixing of the metal bath;
- the need to use electromagnetic stirring of the metal to speed up the process;
- relatively low efficiency of reducing the oxygen content in the metal;
- metal carburization - exposure under white slag is accompanied by an increase in the amount of carbon in the metal by 0.02 - 0.04%, under weakly carbide - by 0.03 - 0.06%, under carbide - up to 0.1%;
- the transition into the composition of the metal of other elements used to deoxidize the slag.
When it is carried out in a steel-smelting unit, the phosphorus of the slag is completely reduced and it passes into metal, therefore, deep extraction deoxidation is possible only when steel is melted in electric arc furnaces. In addition, this principle is used in the processing of steel smelted in any unit with synthetic slags.
1.2.3. Vacuum carbon deoxidation
Vacuum-carbon deoxidation consists in a significant shift of the reaction [C] + [O] = (CO) to the right by reducing the partial pressure in the gas phase by exposing the metal to vacuum or an inert gas. To obtain the basic mathematical dependence, the equilibrium constant equation is solved with respect to the oxygen content in the metal:
This dependence for 1600°C is graphically represented by dashed lines in Fig. . 1.3, which shows how the deoxidizing ability of carbon increases significantly with a decrease. For example, with a residual carbon content of 0.1% already in the case, the equilibrium residual oxygen content in the metal is 0.002%, which is higher than the deoxidizing ability of titanium. At , the deoxidizing ability of carbon can be higher than that of aluminum and even zirconium. However, the data in Fig. 1.3 refer to ideal conditions where the deoxidized metal is not in contact with any oxide phase, such as the ladle lining. This can be observed only in the processes of special remelting (vacuum-arc, electron-beam and plasma-arc).
In conventional steelmaking processes, the metal during vacuuming and gas blowing is constantly in contact with the lining of the ladle or unit and slag, consisting of various oxides. Under these conditions, an increase in the deoxidizing (reducing) ability of carbon leads not only to the deoxidation of the metal, but also to the reduction of the components of oxide phases, for example, to a shift of the reaction to the left as a result of a decrease during carbon deoxidation, therefore, the reaction of oxide reduction begins to affect the residual oxygen content in the metal from the oxide phase. As a result, the degree of deoxidation of the metal by carbon is many times lower than expected, according to Fig. 1.3. These shifts in the reduction reactions of oxides from the lining and slag during carbon deoxidation, in addition to reducing the effect of deoxidation, can lead to an increase in the content of some impurities in the metal to unacceptably high limits.
To reduce the reduction of oxides during evacuation, the fireclay lining of the ladle, which consists mainly of chamotte, is usually replaced with a lining based on stronger oxides, for example, dolomite or magnesite. However, even the adoption of these measures does not make it possible to obtain a residual oxygen content, since the same intense evaporation of iron is observed in high vacuum as in vacuum decarburization, so the effect of carbon deoxidation in the process of evacuation and blowing with an inert gas under normal conditions is only partially used. Nevertheless, even this partial use of increasing the deoxidizing power of carbon makes it possible to noticeably improve the quality of the steel, since the product of CO deoxidation does not remain in the metal.
1.2.4 Deoxidation in the steel plant
Deoxidation in a steelmaking unit is accompanied by a high waste of deoxidizing elements and is advisable only when introduced into the metal a large number sparingly soluble deoxidizers and the impossibility of their preliminary melting. It is necessary to avoid the introduction of elements with a high chemical affinity for oxygen (.) in a steelmaking plant in which melting is carried out on a highly oxidizing slag, for example, in an open-hearth furnace or an oxygen converter, since such elements can only have a normal deoxidizing effect on the metal after the slag has been deoxidized, which consumes several times more deoxidizer than the deoxidation of steel itself. This also applies to applications such as metallic aluminium. Deoxidation in the aggregate with ferroaluminum is possible, since in this case aluminum mainly deoxidizes the metal. Deoxidation in the aggregate is usually precipitating, rarely extraction.
1.2.5 Ladle deoxidation
Deoxidation in a steel-pouring ladle is the most rational way and can be precipitating, extraction and vacuum-carbon or combined. The most common method is precipitating deoxidation in a ladle, as this achieves economy of deoxidizers and reduces the duration of melting. Almost all alloys introduced into steel for deoxidation have time to dissolve and fairly evenly distribute in the metal during the release of the melt from the steelmaking unit into the ladle. As shown by many studies of recent years, deoxidation in the ladle also does not lead to a noticeable decrease in the quality of steel (in terms of the content of non-metallic inclusions in it). It is unacceptable to introduce into the ladle a large amount of alloying additives in the form of sparingly soluble solid materials, since in this case there is an uneven distribution, and sometimes their incomplete dissolution in the metal. This can lead not only to a deterioration in the quality of steel, but also to marriage. In such cases, deoxidation of alloying in the ladle is possible only with preheated or molten ferroalloys.
1.2.6 Deoxidation in the mold
Deoxidation in the mold as an independent method of complete deoxidation of steel is not used: it is usually used for additional deoxidation of semi-calm and very rarely boiling steel. In this case, aluminum is used as a deoxidizer, the consumption of which is determined by the behavior of the metal in the mold. Correction of deoxidation in the mold should be avoided, as this can degrade the quality of the steel and complicate the organization of work in the casting department. However, in some cases deoxidation-alloying in the mold is preferred or even unavoidable. Thus, the deoxidation-alloying of REM is preferably carried out in the mold, with siphon pouring, REM is introduced into the center one, which reduces waste. Alloying with lead is possible only in the mold, since with early introduction (for example, into a ladle), it forms an independent phase, since it is limitedly soluble in liquid iron.
- Features of steel deoxidation by aluminum
- Theoretical foundations of aluminum deoxidation
Aluminum is a very strong deoxidizing agent and is used in the production of calm steels. Additives of aluminum to the metal make it possible to completely calm the steel and avoid the occurrence of porosity in ingots and castings due to carbon oxidation and the release of carbon monoxide bubbles.
Questions of studying the deoxidizing ability of aluminum have attracted the attention of researchers for many years. However, their solution encounters a number of difficulties, mainly due to very low equilibrium concentrations of aluminum and oxygen, which are smaller than the analysis errors. Therefore, the first attempts to determine the deoxidizing ability of aluminum were based on thermodynamic calculations of the equilibrium constant. Taking into account the deviation of the solution of oxygen and aluminum in liquid iron from ideal, Chipman obtained at.
In later studies of the deoxidizing ability of aluminum at and . Consequently, according to experimental data, the deoxidizing ability of aluminum turned out to be significantly lower than calculated. This, apparently, is explained by the fact that the experiments were carried out under an oxidizing slag containing a large amount of ferrous oxide. Some error is also due to the fact that the authors in the calculations took into account not the activity of the reacting substances, but the concentrations.
Taking into account the activities of the reacting elements, the deoxidizing ability of aluminum was studied by Goksei and Chipman. Pure electrolytic iron was melted in an alundum crucible made of pure aluminum oxide in induction furnace with continuous maintenance of an atmosphere of hydrogen and water vapor of a controlled composition. The melt was kept at a constant temperature until equilibrium was reached (usually after aluminum additives) for, and then it was cooled as quickly as possible by lowering the crucible and the cold zone of the furnace and blowing with cold hydrogen, and the content of aluminum and oxygen in the metal was determined.
Thus, in the work, the equilibrium conditions for systems are studied, the reactions in which can be described by the equations:
According to the data received, works were installed orratios of equilibrium concentrations - "apparent" equilibrium constants:
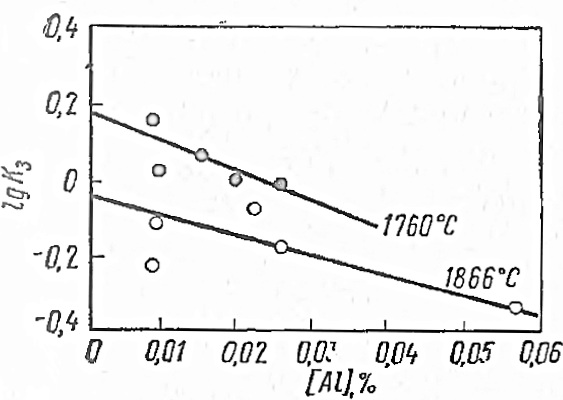
Figure 1.5 - The influence of the aluminum content in the metal on the change
The experimental values are shown in Figs. 1.5. Assuming that for binary solutions the oxygen activity coefficient is a constant value, the authors explained the change; in the ternary system by the influence of aluminum. In this case, the slopes of the lines in Fig. 1.5 represent the interaction parameter, which, within the limits of constancy, can be written as an equation. The found value for equals respectively
As applied to a solution of oxygen and aluminum in iron, the equation takes the form: allowed us to determine the effect of oxygen on the activity of aluminum and calculate and, respectively.With according to the data , equals respectively.
Based on the data obtained and taking into account the activity coefficients, the authors calculated the equilibrium constant for aluminum deoxidation:
The equation of the equilibrium constant, expressed in terms of the activities of the components, differs from the product of equilibrium concentrations. But, as calculations show, the difference in their magnitude is insignificant. the means are equal, respectively, and the means are equal.
The results of studies of the deoxidizing ability of aluminum at, according to different authors, are shown in fig. 1.6.
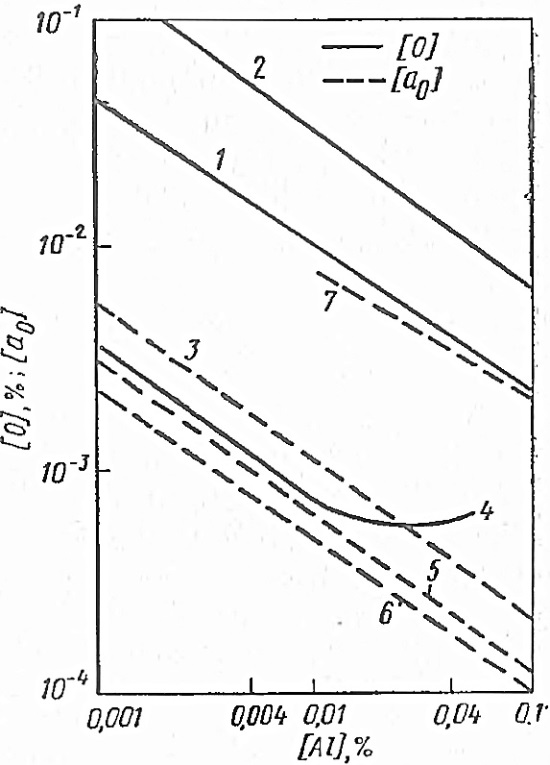
Figure 1.6 - The deoxidizing ability of aluminum at 1600 according to different authors:
The most reliable are the data obtained by Goksen and Chipman during melting in a controlled atmosphere and under conditions that guarantee the achievement of equilibrium, so their equation can be recommended for calculations. It should be noted that, according to the data, the product of equilibrium concentrations differs little from the value of the equilibrium constant, at least at a low aluminum content in iron (Fig. 1.6, curves 4 and 5); therefore, to calculate the equilibrium constant of the reaction of deoxidation of iron with aluminum, you can use the following equation:
However, the formation in the process of deoxidation occurs only with an excess of aluminum in the reaction zone. As the experiments described in the next chapter have shown, with an excess of oxygen, the formation of hercynite () or a melt of variable composition can occur.
The equilibrium constant of the hercynite formation reaction was determined by McLean and Ward:
Together with G.G. Mikhailov, using the method developed by him, we tried to generalize the available results of thermodynamic studies of reactions occurring in the system, taking into account the possibility of formation, and a melt of variable composition. For this purpose, the equations of equilibrium constants of reactions, as well as the equation obtained from the equation, taking into account the heat of melting of corundum and the melting temperature
Equations are received:
The results of calculations according to these equations are plotted on a three-dimensional diagram - the deoxidation diagram (Fig. 1.7). In this diagram, the line described by the equation characterizes the equilibrium of a metal melt containing aluminum and oxygen with solid corundum and hercynite; line ob [equation(38)] - with solid hercynite and liquid slag; line os [equation (39)] - with solid corundum and liquid slag. These lines are the lines of intersection of the surfaces of bivariant equilibrium: the surface I determines the concentration of oxygen and aluminum in the metal in equilibrium with slag, II - with hercynite, III - with corundum.
The maximum number of phases that can be in equilibrium in the system under study is four - three oxide phases and a metal melt. Therefore, on the deoxidation diagram there should be an invariant intersection point of three lines of monovariant equilibrium. The coordinates of this point (0) can be determined from equation (40) obtained by combining equations (37) and (39):
On fig. 1.7 also shows the projection of the spatial diagram on the plane of the trains. Each isotherm on this projection is a curve characterizing the deoxidizing ability of aluminum.
If we consider, for example, an isotherm at 1600°C, then the section de characterizes the equilibrium of the metal with slag, point e—with slag and hercynite, area ef - with hercynite and corundum, fg - with corundum.
Liches o"a", o"b" and o"c" characterize the metastable equilibria of the metal, respectively, with hercynite and corundum, hercyniteand liquid slag, corundum and liquid slag. As will be shown below, metastable reaction products are often formed in the course of deoxidation.

Figure 1.7 - Diagram of the deoxidation of iron by aluminum
Therefore, the metastable equilibrium lines have importance to study the conditions of deoxidation. When considering them, it is clear that under the conditions of deoxidation with aluminum, the formation of oxide phases consisting of hercynite or oxide melt is possible.
1.3.2. Goals and objectives of preliminary deoxidation
BOF steel before deoxidation contains the same amount of oxygen as open-hearth steel at the same carbon concentration, and the conditions for its deoxidation are not fundamentally different. Therefore, the described methods for the deoxidation of open-hearth steel are generally also characteristic of the oxygen-converter process, which makes it possible to shorten the description of the basics of the technology for the deoxidation of converter steel. At the same time, the conditions for the deoxidation of converter steel differ in some features that affect the applicability of individual methods and their development.
In an oxygen converter, after the end of the purge during preliminary deoxidation, no oxidation of the metal occurs due to the supply of oxygen from the atmosphere, as is the case in an open-hearth furnace. There is only some oxidation due to slag, and at a small surface of its contact with the metal. This reduces the waste of deoxidizers, eliminates the re-boiling of the bath during pre-deoxidation in the converter, and facilitates obtaining the desired carbon content.when draining without prior deoxidation. The possibility of preliminary deoxidation in the converter is also facilitated by the fact that during its implementation there is no increase in the hydrogen content in the metal.
However, pre-deoxidation in the converter causes reduction of phosphorus, which requires good dephosphorization before deoxidation. Additive to the converter of ferroalloys for deoxidation, as well as their addition to the ladle, leads to a decrease in the temperature of the metal. Therefore, to compensate for heat losses, it is necessary to increase the temperature of the metal by the end of blowing on with the introduction of ferroalloys.
Taking into account the noted features, two methods of deoxidation of oxygen-converter steel are used:
- with preliminary deoxidation in the converter and final deoxidation in the ladle;
- with deoxidation only in the bucket.
Preliminary deoxidation in an oxygen converter is mainly used to remove secondary oxidation to reduce the subsequent waste of the main mass of ferroalloys. This requires a deep dephosphorization of the metal and the removal of part of the slag with the introduction of a new one to prevent the possibility of a significant reduction of phosphorus.
Pre-deoxidation in the converter is usually carried out with aluminum, silicomanganese or ferrosilicon. The final deoxidation is carried out in a ladle with ferrosilicon and aluminum.
Relatively large debt of the oxygen converter in the preliminary deoxidation, the duration of which is up to 50% or more of the blowdown time, the possibility of phosphorus recovery and the associated complication and elongationprocess if it is necessary to work on two slags. The increased waste of deoxidizers limits the use of pre-deoxidation in BOF smelting.
In the smelting of low- and medium-alloy steels, deoxidation and alloying with oxidizing elements (chromium, vanadium, etc.) have become more widespread only in the ladle. As noted, it is easier to do this when melting in oxygen converters than when melting in open-hearth furnaces, due to the absence of intense oxidation, in particular carbon, after blowing is completed.
Deoxidizers and alloying agents are usually added to the ladle while the metal is being drained. In the case of adding a large amount of alloys, some of them are loaded into the ladle before draining, the rest into the jet
Due to the advantages of metal deoxidation and alloying in the ladle, the use of exothermic ferroalloys is promising for the production of alloyed steels in converters.
- Deoxidation of low carbon boiling steel
Aluminum is usually added to the ladle in quantity. The amount of aluminum introduced increases with a decrease in the carbon content in the steel and an increase in the content of ferrous oxide in the slag and depends on the casting method. In siphon casting, when the rate of metal rise in the molds is less than in pouring from above, the need for aluminum deoxidation is greater. When pouring from above, especially high-speed, it is even sometimes necessary to introduce boiling intensifiers into the metal, for example, a mixture of mill scale (with fluorspar and soda ash or sodium nitrate.
In siphon casting, even a slight peroxidation of the metal immediately manifests itself during the casting itself, when, due to boiling, the steel splashes out of the center and it is necessary to slow down the metal stream from the ladle. In this case, aluminum () is placed under the jet, in the center.
The deoxidation of boiling steel with aluminum is most widely used in the smelting of low-carbon high-quality steel 08kp used for the production of thin sheets, SAE 1008, SAE 1006 - for wire rod. In this case, the amount of aluminum planted in the metal is chosen taking into account the oxidation of the metal and the technological parameters of melting and casting.
V. A. Efimov and V. N. Sapko, taking into account the formula they obtained, determined the consumption rates of aluminum placed in a ladle during high-speed casting of steel. With an increase in the content of ferrous oxide in the slag c and the temperature at the outlet c, the amount of aluminum proposed by them increases with
I.S. Marakhovsky and Yu.S. Furman, on the basis of calculations, taking into account the need to obtain a dense crust with a thickness and conditions for the formation of bubbles, found the optimal oxidation of steel 08kp (0.07–0.09% C in a ladle sample) and the necessary aluminum additives in the ladle at different casting speeds with a siphon (A, B) and top (B):
|
Pouring option |
A |
B |
V |
|
Filling speed, m/min |
0,25 |
0,45 |
1,6 |
|
0,027-0,033 |
0,03-0,036 |
0,037-0,048 |
|
|
Amount of aluminum, g/t |
100-200 |
70-160 |
10 |
The experience of the Zaporizhstal plant with siphon casting according to option A and statistical processing show that the indicated consumption of aluminum makes it possible to obtain cold rolled sheet satisfactory quality.
At MMK, to obtain a healthy ingot of 08kp steel, the oxidation of the metal is regulated by aluminum, which is added in two stages: the main part in the form of ingots into the ladle and the rest in the form of shot into the mold (pouring from above).
The amount of aluminum planted in the ladle is determined by an empirical formula obtained on the basis of statistical processing of production data:
where: - aluminum consumption,
– steel temperature before release, °C.
The amount of shot put into the mold is determined by the casting master, according to the behavior of the metal in the mold. Usually it is equal. A sign of satisfactory deoxidation of steel is the growth or shrinkage of the ingot by no more than. This results in a dense crust.
The experience of MMK has shown that the addition of aluminum to the bottom of the mold during casting of steel 08kp, causing excessive deoxidation of the first small portions of the metal, leads to significant shrinkage and causes bubbles to be located close to the surface of the ingot. The aluminum additive at the end of filling eliminates shrinkage, but does not provide a dense crust in the lower part of the ingot (porous crust). Only a uniform introduction of aluminum provides a high-quality ingot.
A significant part of the boiling steel is obtained with mechanical or chemical plugging after or during casting. When plugged, boiling stops and a steel is obtained that favorably differs from boiling steel by the lower chemical inhomogeneity of the ingot, the increased density of rolled products and the high yield due to the smaller trimming of the upper part of the ingot. At the same time, an ingot of corked steel is not inferior to a boiling steel in terms of surface quality, since the boiling metal is poured and begins to crystallize in the mold.
Mechanical sealing is usually carried out when pouring into bottle molds with a narrow neck, which is covered with a lid beforehand or when the metal approaches. Boiling stops due to the formation of a hard crust upon contact of the metal with the lid. The disadvantages of mechanical plugging (difficulties in maintenance of bottle molds, difficulty of pouring from above, small thickness of the dense outer skin of the ingot during early lidding) limit its use.
Chemical plugging is carried out by introducing deoxidizers into the metal at the end of mold filling or a few minutes after filling. Usually, aluminum or sometimes finely crushed ferrosilicon (45% or 75%) is used for this. When they are introduced into the mold, due to a slowdown in the carbon oxidation reaction and a decrease in metal circulation in the upper part of the ingot, the steel solidifies with the formation of a dense "bridge" that prevents the release of gases. The pressure under the bridges increases and the boiling stops completely.
The authors, considering the conditions of chemical plugging as balancing the pressure of gases inside the ingot by the resulting "bridge", found that the consumption of aluminum for plugging is determined mainly by the carbon content in the steel and, to a lesser extent, by the parameters of the ingot and casting technology (Fig. 1.8).

Figure 1.8 - Dependence of aluminum consumption for plugging boiling steel ingots on carbon content (numbers near the points, number of melts)
According to the data, when casting St.Zkp steel with a siphon into ingots weighing 7 tons with the introduction of aluminum into the center, when the metal level is below the required one, the optimal consumption of aluminum is (depending on the composition and temperature). The optimal consumption of aluminum corresponded to the convex or bumpy shape of the head part of the ingots.
With siphon casting, aluminum can also be introduced onto the surface of the metal in the mold, but its consumption increases by about 10% due to oxidation by slag and air. Whenpouring aluminum from above give to the surface of the metal through after filling the mold. Then the upper layers are mixed with wooden or steel bars.
- STATEMENT OF OBJECTIVES IN WORK
In the course work, the tasks are formulated:
- Explore the technology of preliminary and final deoxidation of mild steel SAE 1008;
- choose the most optimal from the point of view of economy type of supplied deoxidizer (liquid, pig, granulated);
- Reducing the specific consumption of the deoxidizer.
- Reducing the cost of steel,quality improvement.
- CONCLUSIONS ON COURSE WORK
- By doing term paper, it was determined that deoxidizing elements, in addition to their main function of reducing the residual oxidation of metal after smelting, have a number of positive qualities, such as:
– structure refinement, which contributes to strength increase;
- bind and neutralize Negative influence nitrogen, sulfur;
– minimize the effect of non-metallic inclusions remaining in the metal, binding them into strong connections globular shape, which are evenly distributed in the volume of the metal and are slightly deformed during rolling.
- The most common and optimal in world practice is the precipitating method of deoxidation in a ladle; this contributes to the saving of expensive ferroalloys, their more complete use, which leads to a reduction in cost. finished products which is always relevant.
- It has been established that of the deoxidizers available on the market, taking into account prices, in the production of steel SAE 1006, it is optimal to use for final deoxidation (in a ladle) - since its consumption is less (against) a ton of steel, waste is lower, the effect is higher. hashigh chemical affinity to three harmful impurities at once - oxygen, nitrogen and sulfur, and reduces their harmful effect with residual hundredths of a percent in steel, against the same - reduces the harmful effect only S , with a residual content, but with all this, it is very expensive and a lot of it passes into slag in the form ofpreliminary deoxidation (in a steelmaking unit).
LIST OF USED LITERATURE
- Yavoisky, A.V. Scientific foundations of modern steelmaking processes [ Text ] / A.V. Yavoisky, P.S. Kharlashin, T.M. Chaudhry. - Mariupol. - 2003. - 276 p.
- Bigeev, A.M. steel metallurgy. Textbook for universities - 2nd ed., Revised. and additional[ Text ] / A.M. Bigeev. - Chelyabinsk branch. – M.: – Metallurgy. - 1988. - 480 p.
- Kulikov, I.S. Deoxidation of metals [ Text ] / I.S. Kulikov. – M.: Metallurgy. - 1975. - 504 p.
- Savostin, D.Z. Open-hearth steel production[ Text ] / D.Z. Savostin. – M.: Metalurgizdat. - 1961. - 288 p.
- Knuppel, G. Deoxidation and vacuum treatment of steel.Ch. P. Fundamentals and technology of ladle metallurgy[ Text ] / G. Knupel, Translated from German. – M.: Metallurgy. - 1984. - 414 p.
- Bornatsky, N.N. Physico-chemical basics steelmakingprocesses [Text] / N.N. Bornatsky. – M.: Metallurgy. - 1974. - 320 p.
- Baptizmansky, V.I. Steel-smelting work: Navch. Helper[Text] / V.I. Baptizmansky, B.M. Boychenko, O .G. Velichko ta in .. - K .: ІЗМН. - 1996. - 400 p.
- Boychenko , B.M. Steel milling converter[Text] / B.M. Boychenko, V.B. Okhotsk , P.S. Kharlashin. - Dnipropetrovsk: RVA. - 2004. - 454 p.
- Povolotsky, D.Ya. Steel deoxidation[ Text ] / D.Ya. Povolotsky. – M.: Metallurgy. - 1972. - 208 p.
- Chipman, J.J. Transt. amer. soc. / J.J. Chipman // Metals. - 1934. - v.22. – P.385.
- Wentrup, H. Techn. Mitt. Krupp. / H. Wentrup, G. Hieber // Forschungsbericht. - 1939. - Bd. - 1. - No. 2.– S.47.
- Hilty, D.C. Trans. Metallurg. soc. / D.C. Hilty, W.Crafts //AIME. - 1950. - v.585. – p.413.
- Gokcen N.A. / N.A. Gokcen, J.J. Chipman // Metals. - 1953. - v.5. – P.137.
- Mikhailov, G.G. Proceedings of the Academy of Sciences of the USSR [ Text ] / G.G. Mikhailov, D.Ya. Povolotsky // Metals. - 1971. - No. 6. - p.7.
- Efimov, V.A. [ Text ] / V.A. Efimov, V.N. Sapko // Steel. - 1969. - No. 9 P. 785.
- Kovalev, G.M. Proceedings of universities [ Text] / G.M. Kovalev and others // Ferrous metallurgy. - 1969. - No. 4. - P.42.
- Levin, S.L. Proceedings of universities [ Text] / S.L. Levin // Ferrous metallurgy. - 1969. - No. 8. - P.44.
As well as other works that may interest you |
|||
| 32357. | General concept of temperament. Properties and types of temperament, their manifestation in activity and behavior | 16.91KB | |
| temperament congenital individual characteristics of a person, which determine the dynamic characteristics of the intensity and speed of response, the degree of emotional excitability and balance, the features of adaptation to environment. They determine the dynamics of various human activities, play, educational, labor, recreational: Reactivity is the degree of involuntary reactions of a person to external or internal influences of the same strength. Plasticity, ease, flexibility and speed of adaptation of a person to changing external ... | |||
| 32358. | Self-consciousness of the individual. The structure of self-consciousness. The development of self-consciousness in ontogenesis | 18.56KB | |
| Thus, self-consciousness includes: Self-knowledge intellectual aspects of self-knowledge Self-attitude emotional attitude towards oneself In general, three layers of human consciousness can be distinguished: Attitude towards oneself Expectation of other people's attitude towards oneself attribute projection Attitude towards other people: egocentric level of relations if they help me then it good people group-centric level if he belongs to my group then he is a good pro-social level do to others as you would like them to do to you... | |||
| 32359. | General concepts of character. Character structure. Character typology | 13.96KB | |
| Character structure. Typology of character. In the structure of the personality of character, it occupies a central place, combining all other properties and behavioral features: Influences cognitive processes On emotional life On motivation and will Determines the individuality and originality of the personality The character of a person is an alloy of innate properties of higher nervous activity with individual traits acquired throughout life. Character structure: Traits expressing the orientation of the personality, stable needs of installation, interests, inclinations, ideals, goals ... | |||
| 32360. | Group and joint activities. Factors of effectiveness of group and joint activities | 15.38KB | |
| Factors of effectiveness of group and joint activities. Compatibility The ability of group members to work together. Types of compatibility: Psychophysiological certain similarity of characteristics of people and, on this basis, the consistency of their emotional and behavioral reactions, synchronization of the pace of joint activities. Evaluation criteria: Performance results. | |||
| 32361. | Psychological readiness of the child for school. Methods for diagnosing psychological readiness for schooling | 13.85KB | |
| The psychological readiness of the child for schooling is the necessary and sufficient level of mental development of the child for mastering the school curriculum in the conditions of learning in a group of peers. The structure of the component: Psychomatory readiness balance of the processes of excitation and inhibition, which allows the child to focus his attention for a longer time, contributes to the formation of arbitrary forms of behavior and cognitive processes; the development of small muscles of the hand and hand-eye coordination, which creates ... | |||
| 32362. | Test method | 19.05KB | |
| Tests differ from other research methods in that they imply a clear procedure for collecting and processing primary data, as well as a kind of subsequent interpretation. It became widely used in psychology after the publication in 1980 of Catell's work Mental Tests and Measurements, which was devoted to the test results of US students. Types of tests: According to the characteristics of test values: verbal non-verbal According to the form of conducting: group individual Depending on the presence or absence of time restrictions: speed ... | |||
| 32363. | The development of marital relations. Psychological aspects of relationships in marriage. Dynamics of sexual relations in marriage | 16.21KB | |
| Family models: Parental model An individual learns marital behavior based on the identification of parents of the same sex. Based on the behavior of parents of the opposite sex, an idea of how a partner should behave is built. In marriage, each of the partners is trying to adjust their real relationship with the reference. Harmonious relationships become possible only if the partner with his internal program resembles the parents of the opposite sex. In this case, there is a transfer of connections that existed in ... | |||
| 32364. | Psychological foundations of education. Organization of education, taking into account the specifics of various categories of children | 13.68KB | |
| The psychology of education teaches the internal mechanisms of the formation and development of the personality as a whole, as well as its individual properties. Social upbringing is the purposeful creation of material spiritual conditions organized for human development, this is a purposeful activity designed to form in children a system of personality traits of views and beliefs. Goals of education: Education of a comprehensively and harmoniously developed personality that combines spiritual wealth, moral purity and physical perfection. Raising a Socially Competent... | |||
| 32365. | Research methods in psychology | 14.05KB | |
| A method is a way by which the subject of science is known; it is a way of obtaining facts about the manifestations of the psychological characteristics of a person. Being a means of studying the facts and laws of the human psyche, a particular method relies on the basic laws of its development and functioning and is based on the methodology of the science in which it is used, and methodology is a set of principles. The human psyche is a complex psychological system in which all processes and functions are closely interconnected. is there... | |||
Metal, which consists in removing oxygen from the liquid metal, which is present in the form of oxides, by adding deoxidizers (reducing agents) to the metal - substances that have the ability to combine with oxygen. Their quality largely depends on the deoxidation of metals. Good deoxidizers are C, Si, Mn used in the form of ferroalloys, including complex deoxidizers (silicomanganese, silicocalcium and others). Deoxidation products float to the slag or are removed in the form of gas (carbon monoxide).
ICM (www.website)
Recovery process- the physicochemical process of obtaining metals from oxides by splitting off and binding oxygen with a reducing agent - a substance capable of combining with oxygen. A typical reduction process is the blast furnace process, in which iron is reduced from ores mainly with carbon or its oxide.
Steel deoxidation
Steel deoxidation is a reduction in the oxygen content in steel to a level that excludes the possibility of oxidative reactions in the ingot. The resulting solid, liquid or gaseous deoxidation products steels must be removed before the ingot solidifies, as they reduce the quality of the steel. The oxygen content after steel deoxidation decreases by an order of magnitude.
Stages of the deoxidation process:
- Dissolution of deoxidizers in liquid metal.
- Reactions between oxygen and deoxidizer.
- Embryo formation, growth and isolation of deoxidation products.
Steel deoxidation methods:
- Precipitating deoxidation;
- Diffusion deoxidation;
- Special methods of deoxidation (treatment with synthetic slags; deoxidation in vacuum).
Precipitating deoxidation
Such a deoxidation method as precipitating deoxidation is carried out using elements that have a greater affinity for oxygen than Fe. Depending on the situation, manganese, silicon, aluminum or complex deoxidizers are used as deoxidizers.
Diffusion deoxidation
The expression "diffusion" does not quite correspond to the essence of the process of this deoxidation method. A more precise term is "extraction deoxidation". During diffusion deoxidation, the oxygen content decreases due to slag deoxidation. Deoxidizers can be C, Si, Al. The main task is to reduce FeO in the slag, which enhances the diffusion of oxygen from the metal into the slag (the Nernst distribution rule).
ICM (www.website)
This method of deoxidation is used only in arc furnaces, where there are no burning gases.
Processing with synthetic slags (deoxidation method)
The treatment of molten iron with synthetic slags is widely used in practice. In an arc furnace, slag is introduced from Al 2 O 3 and CaO; slag is poured into a ladle, and a jet of metal from the furnace is poured into the same place from a height of 3-6 m. This method of deoxidation reduces the content of oxygen and sulfur.
Electroslag remelting (deoxidation method)
The main purpose of electroslag remelting (ESR) is the purification of steel from sulfur and non-metallic inclusions in the process of melting the source material in a heated slag bath. In addition, due to solidification in a water-cooled mold, it is possible to control the structure of the ingot.
Vacuum deoxidation
Vacuum deoxidation is based mainly on the decarburization reaction, since the deoxidizing power of carbon increases significantly in vacuum.
Review author: Kornienko A.E. (ICM)
Lit.:
Converter steel deoxidation is carried out by the sedimentation method in the ladle during tapping. Deoxidizing agents are not introduced into the converter in order to avoid their large waste.
Quiet steels usually deoxidize manganese, silicon and aluminum, titanium, calcium and other strong deoxidizers are additionally used on certain steel grades. Boiling steel is deoxidized with manganese alone.
In old workshops that do not have out-of-furnace processing units, all deoxidizers are introduced into the ladle during the release, usually starting with weaker ones (having a lower chemical affinity for oxygen), and then stronger ones are introduced, which reduces their waste.
The sequence of introducing widely used deoxidizing alloys into the ladle is as follows: ferromanganese or silicomanganese is introduced first, then ferrosilicon and lastly aluminum. Boiling steel is deoxidized with one ferromanganese. The supply of deoxidizers begins after filling the ladle with liquid metal by about 1/4-1/3, and ends when it is filled with metal by 2/3, which makes it possible to avoid the ingress of deoxidizers into the slag and their increased waste. The amount of manganese and silicon introduced into the metal is calculated so that not only deoxidation is ensured, but also the content of these elements required in a given steel grade is obtained.
When determining the consumption of deoxidizers, it is taken into account that at deoxidation of calm steel and the introduction of deoxidizers into the ladle, their waste is: manganese 10-25%, silicon 15-25%. When deoxidizing boiling steel, manganese waste is 20-35%. The consumption of aluminum for deoxidation, depending on the carbon content in the smelted steel, is 0.15-1.20 kg per 1 ton of steel, increasing with a decrease in the carbon content; most of the introduced aluminum (60-90%) burns out. The converter slag that enters the ladle at the end of the metal tapping is thickened at many plants with additives of lime or dolomite in order to reduce the oxidation of the additives introduced into the ladle by slag iron oxides and the reduction of phosphorus from the slag.
In modern converter shops equipped with installations for finishing liquid steel in a ladle, only a part of deoxidizers is introduced into the ladle during the release of metal - mainly weakly oxidizing, i.e. having a not very high affinity for oxygen (ferromanganese, silicomanganese and less often ferrosilicon). To prevent converter slag containing phosphorus and iron oxides from entering the ladle, it is cut off at the end of the tapping, and materials (granulated blast-furnace slag, vermiculite, a mixture of lime and fluorspar, etc.) are loaded into the ladle. to create a slag cover which protects the metal surface from oxidation and cooling.
Then the ladle is transported to the steel finishing plant, where ferrosilicon, aluminum and, if necessary, other strong deoxidizers are introduced into the metal in the process of stirring purging with argon; according to the results of the analysis of samples taken during out-of-furnace processing, the content of silicon and manganese in the metal is adjusted, which ensures guaranteed obtaining of a given steel composition. For better assimilation of aluminum, it is desirable to introduce it into the bulk of the metal using submerged rod or in the form of a wire fed into the bucket from above at high speed using a tribe device.
The cut-off of slag in order to prevent it from entering the steel-pouring ladle during the release of metal is done in several ways. The simplest of them - fast lifting of the converter at the moment of the end of the metal drain- is not effective enough. Another way is to cut off with steel balls in a refractory shell: at the end of the release, the ball is introduced into the converter, where it floats on the slag-metal border and, together with the last portions of the metal, enters the taphole channel, blocking it. More efficient ways to forced closure of the entrance: a sliding slide gate fixed on the casing of the tap-hole and moved by a hydraulic drive; pneumatic device, which is a cast-iron nozzle, fixed with a bracket on the converter body. At the right moment, the nozzle, through which air flows under pressure, is inserted into the taphole channel from below by turning the bracket, while the locking effect is created by compressed air.
Steel is deoxidized in two ways: precipitating and diffusion.
Precipitating deoxidation It is carried out by introducing into liquid steel soluble deoxidizers (ferromanganese, ferrosilicon, aluminum) containing elements that have a greater affinity for oxygen than iron.
As a result of deoxidation, iron is reduced and oxides are formed: MnO, SiO 2 , Al 2 O 5 , which have a lower density than steel and are removed into the slag.
Diffusion deoxidation carried out by deoxidation of the slag. Ferromanganese, ferrosilicon and aluminum in crushed form are loaded onto the surface of the slag. Deoxidizers, reducing iron oxide, reduce its content in the slag. Consequently, the iron oxide dissolved in the steel turns into slag. The oxides formed during this process remain in the slag, and the reduced iron passes into steel, while the content of non-metallic inclusions in the steel decreases and its quality increases.
Depending on the degree of deoxidation, steels are smelted:
Quiet - calm steel is obtained by complete deoxidation in the furnace and ladle.
· boiling - boiling steel is not completely deoxidized in the furnace. Its deoxidation continues in the mold when the ingot solidifies due to the interaction of iron oxide and carbon: FeO + C = Fe + CO. The resulting carbon monoxide CO is released from the steel, helping to remove nitrogen and hydrogen from the steel, gases are released in the form of bubbles, causing it to boil. Boiling steel does not contain non-metallic inclusions, therefore it has good ductility.
semi-calm - semi-calm steel has an intermediate deoxidation between calm and boiling. Partly it is deoxidized in the furnace and in the ladle, and partly in the mold, due to the interaction of iron oxide and carbon contained in the steel.
Alloying of steel is carried out by introducing ferroalloys or pure metals in the required amount into the melt. Alloying elements whose affinity for oxygen is less than that of iron (Ni, Co, Mo, Cu) do not oxidize during melting and pouring, so they are introduced at any time during melting. Alloying elements whose affinity for oxygen is greater than that of iron (Si, Mn, Al, Cr, V, Ti) are introduced into the metal after deoxidation or simultaneously with it at the end of the melt, and sometimes into the ladle.
To provide the foundry and rolling shop with prepared scrap metal in the amount of 1,110 thousand tons per year, it is planned to build a scrap preparation site (STP).
The lime-burning shop is designed to provide electric-steel-smelting production of the CRC with metallurgical lime.
The capacity of the complex of the lime-calcining shop is accepted with a margin compared to the need of the rolling mill, which predetermines the stable supply of this shop with metallurgical lime;
some excess lime will be sold as marketable products.
At the same time, it should be borne in mind that the Cimprogetty type furnace allows, within a fairly wide range (70 ÷ 120% of the rated power), to regulate its productivity depending on the needs of adjacent production.
To provide the HRC with prepared scrap metal in the amount of 1,110 thousand tons per year, it is planned to build a scrap preparation department (STP). Steel smelting in the rolling mill is carried out according to three options for melting charge (depending on the group of steel grades being smelted):
– option I: 100% scrap metal;
- option II: 80% of scrap metal; 20% pig iron;
- option III: 40% of scrap metal; 25% pig iron; 35% metallized briquettes.
The size of pieces of scrap metal sent to the rolling mill should be no more than 1.5x0.5x0.5 m. Weight - no more than 1 ton.
According to the technology of steel smelting in EAF, the total volume of heavy scrap should not exceed 60% of the total mass of the charge.
Annual work fund technological equipment Department adopted 300 days.
The department receives: circulating scrap (trimmings from ONRS and rolling shop, scrap from ONRS), prepared and unprepared steel scrap and cast iron from outside.
The structure of the scrap preparation department provides for the construction of two spans - unprepared and prepared scrap. The spans are equipped with overhead special magnetic cranes with a lifting capacity of 32 tons on a traverse in the amount of 8 pcs (4 pcs in each span). Elevation of crane rails in spans is +16.0 m. Each crane is equipped with two removable magnets of DKM200TA type. Scrap load capacity of each magnet is 2.5÷3.0 t.
In the span of unprepared scrap, it is planned to organize two sections for flame cutting of oversized scrap with manual gas cutting posts.
CONCLUSION
The CRC was the first in Russia to master the production of hot-rolled products from thin slabs (70 and 90 mm thick) using the most economical technology based on the combination of continuous casting and rolling in a single technological process.
The casting and rolling complex is one of the first in Russia industrial facilities, environmental characteristics which fully comply with the requirements of the European Union. The indicators of emissions from the activities of the forestry complex into the atmosphere are less than 5 mg per 1 cubic meter which is well below current environmental standards.
APPENDIX
Annex A
Figure A - Equipment layout plan
 Annex B
Annex B
Figure B - Chipboard scheme
|
|
©2015-2017 site
All rights belong to their authors. This site does not claim authorship, but provides free use.
Metal deoxidation the process of removing from molten metals (mainly steel and other iron-based alloys) oxygen dissolved in them, which is a harmful impurity that worsens the mechanical properties of the metal. For R. m., elements (or their alloys, such as ferroalloys) are used that are characterized by a greater affinity for oxygen than the base metal. So, steel is deoxidized with aluminum, which forms a very strong oxide Al 2 O 3, which is released in the liquid metal in the form of a separate solid phase. The degree of deoxidation, i.e. the final oxygen content in the metal [O]. for example, in the reaction R + O = RO (T), where R and O are the deoxidizer and oxygen in the metal solution, is determined by the concentration of the deoxidizer [R], temperature and strength of the oxide RO. In accordance with the Acting Masses Law
the equilibrium constant of the above reaction has the form its numerical value is the greater, the stronger the oxide, i.e., the more significant the loss of free energy during its formation from the elements and, consequently, less [O] at a given concentration R and temperature. For effective R. m., it is necessary that the deoxidation products do not remain in the steel in the form of non-metallic inclusions (See Non-metallic inclusions). The rate of their ascent to the surface of the liquid bath depends on the temperature and viscosity of the metal, the density of inclusions, and the intensity of flows within the melt. The removal of inclusions is favored by the presence of a liquid slag that assimilates oxides. R. m. is used in some cases in non-ferrous metallurgy (for example, the deoxidation of copper with the help of carbonaceous reducing agents). Lit.: Rostovtsev S. T., Theory of metallurgical processes, M., 1956. L. A. Shvartsman.
Great Soviet Encyclopedia. - M.: Soviet Encyclopedia. 1969-1978 .
See what "Deoxidation of metals" is in other dictionaries:
The process of removing from molten metals (mainly steel and other iron-based alloys) the oxygen dissolved in them, which is a harmful impurity that worsens the mechanical properties of the metal. Elements are used for deoxidation ... ... Wikipedia
Metal deoxidation- removal of dissolved oxygen from liquid metals (mainly steel and other iron-based alloys) in order to improve quality. Deoxidation is often combined with metal alloying. Types of deoxidation: ... ... encyclopedic Dictionary in metallurgy- Deoxidation of metals is the process of removing from molten metals (mainly steel and other iron-based alloys) the oxygen dissolved in them, which is a harmful impurity that worsens the mechanical properties of the metal. For deoxidation ... ... Wikipedia
Metals, removal from molten metals (mainly steel) of the oxygen dissolved in them. Carry out the introduction of chemical elements that form stable compounds with oxygen. For deoxidation. Al, Si, Ti and others are used ... ... encyclopedic Dictionary
One of the main metal refining operation, which consists in removing oxygen from a liquid metal by adding deoxidizers (reducing agents) to the metal, which have the ability to combine with oxygen. Good deoxidizers are ... ... The Big Encyclopedic Polytechnical Dictionary - non-semerization of cast iron, one of the types of redistribution of liquid iron into steel without fuel consumption (see Converter production). B. p. was proposed by G. Bessemer in 1856 in connection with the growing demand for steel, caused by the growth of iron. d… Great Soviet Encyclopedia










Mixed Personality Disorder: Causes, Symptoms, Types and Treatments
GTA 4 control settings
FAQ on Smuggling in GTA Online
LSPDFR - welcome to the police
The huge map of Grand Theft Auto San Andreas and its secrets