The evaporation of metals takes place in completely different ways, depending on the total pressure in the vapor-gas phase above the melt. The total pressure in the vapor-gas phase determines the mean free path of the particles. If the total pressure is high, the free path is very small and, for example, at 10V5 Pa is not more than 10V-4 cm. Under these conditions, the amount of evaporated metal M is expressed by Dalton's empirical law:
where S is the area of the free surface of the melt, from which evaporation takes place; p° is the equilibrium vapor pressure of the metal at a given temperature; p" is the actual vapor pressure of a given metal above the melt; ptot is the total pressure in the vapor-gas phase above the melt; t is the time; k is the proportionality factor.
The value of p" cannot be determined in advance, it depends on the shape of the vessel where the metal is located, the speed of movement of gases over the melt, and other circumstances. In practice, p" is determined empirically. If p "≤p °, the metal is evaporating; if p" ≥ p °, the opposite phenomenon is observed - vapor condensation.
The given Dalton formula shows well the influence of the total pressure in the vapor-gas phase on the evaporation process. As can be seen from the formula, the larger it is, the smaller the amount of evaporated metal. Therefore, by introducing some gas inert for the metal over the melt, it is possible to significantly slow down the evaporation process, although the value of the partial vapor pressure of the metal itself does not change from this. It is known that it is determined only by temperature. If evaporation of any one component X from a liquid alloy is considered, it is necessary to substitute p°xNx instead of p°x in the Dalton formula, where Nx is the atomic fraction of this component in the alloy.
With a decrease in the total pressure over the melt, the mean free path of particles in the gas phase increases accordingly, and when this length becomes comparable with the dimensions of the vessel where evaporation occurs, the process changes radically. Provided that the walls of the vessel are cold, so that almost all the gas particles that have reached them are fixed on them and do not return to the gas phase, it is possible to calculate the amount of evaporated metal. The transition to new patterns of evaporation is observed at a total pressure of no more than 0.133 Pa, i.e., at a sufficiently deep rarefaction. Therefore, this process is called evaporation in a vacuum. It is described by the Langmuir formula:
where M is the mass of evaporated metal during time t from area S at melt temperature T; R is the gas constant; p°A is the vapor pressure of the metal at temperature T, A is the atomic mass of the metal. In most cases, metal vapors, like inert gases, are monatomic.
In the event that the evaporation of metal A in vacuum from a liquid alloy, in which the atomic fraction of this metal is NA, is considered, the Langmuir formula takes the following form:
Since the evaporation of this metal A comes from the solution, the partial vapor pressure of this metal is taken into account, which is equal to the product of the vapor pressure of the pure metal P°A, its atomic fraction in the alloy NA and the activity coefficient γA. In addition, the formula no longer includes just the mass of the evaporated metal, but the evaporation rate, expressed as dm / dt. This is because the alloy base and the metal in question have different atomic masses and different vapor pressures. Therefore, they will evaporate differently. As a result, the content of the considered metal in the melt will begin to change immediately. Only at the very first moment of the evaporation process, the value of NA is known exactly - this is the concentration of the metal in the original alloy.
It should be noted that evaporation acquires practical significance for melting only if the metal in question has a sufficiently high vapor pressure at a given temperature. When evaporation takes place in an environment of other gases at a total pressure of more than 1330 Pa, this phenomenon has to be taken into account if the equilibrium vapor pressure of the metal is more than 100-200 Pa. For the case of evaporation in vacuum at a residual total pressure of less than 0.133 Pa, the process acquires practical significance for the preparation of alloys if the equilibrium pressure of metal vapors exceeds 13 Pa. That is why zinc, magnesium, calcium, manganese and chromium are named volatile.
Boiling of pure metals during melting in foundries is rare. However, this phenomenon is observed when working with alloys, which include volatile components. The phenomenon of boiling of alloys can be considered according to the diagram in Fig. 3. This figure shows the state diagram of system B of metals A and B, which form continuous solid and liquid solutions. In addition to the usual areas of solid, liquid and solid-liquid states, the area of the gaseous state, which lies at high temperatures, is also indicated here. The diagram corresponds to equilibria at a pressure of 10v5 Pa. Therefore, the points tboilA and tboilB are the usual boiling points of these pure metals, above which the metals are in the gaseous state. The diagram has a region of two-phase state liquid - gas. The line tmA - t2 - tmB shows the end temperature of alloy melting. The line - t3 - depicts the temperature at which the alloys begin to boil.
Color marking of steels
Boiling water, with a flurry of bubbles that rise from the surface as the water comes to a boil, is central to most power plants, heating and cooling systems, and desalination plants. This degree of control over the boiling process, regardless of temperature, Wang says, has not previously been demonstrated, despite the ubiquity of boiling in industrial processes. Other systems have been developed to control boiling using electric fields, but they require special fluids, not water, and are thousands of times more high voltages, which makes them uneconomical for most applications.
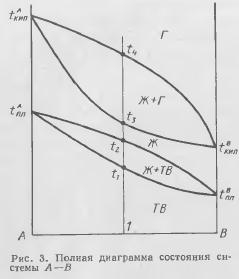
The mutual position of these lines determines the area of existence of alloys in the liquid state. For example, alloy 1 will start to melt at t2, will start to boil at t3, will become completely gaseous above t1. On fig. 3 shows that the temperature region of the liquid state is narrowest in the middle part of the diagram. As can be seen, the initial boiling point of alloy 1, equal to t3, is slightly lower than the melting point of pure component A. This arrangement of the lines of the end of melting and the onset of boiling is due to the fact that component B is much more fusible than component A, and, in addition, the boiling point of pure component B is below the melting point of pure component A, and the boiling point of pure component A is very high. Under such conditions, it turns out that the alloys lying in the middle part of the diagram have an initial boiling point very close to the end point of melting. Therefore, the usual overheating in the preparation of alloys can lead to boiling of the melt. According to fig. 3 can also explain the phenomenon of temporary boiling of the melt when a fusible and highly volatile component is introduced into a liquid, non-volatile one. For example, if you introduce into pure liquid metal A solid metal B, then the latter will not only start to melt, but may also boil, since tboilB≤tmA. boiling up will be short-lived, since the simultaneous dissolution of B into A will lead to the formation of alloys, the initial boiling point of which is much higher. Similar phenomena practically occur when melting brass (copper-zinc alloys).
In the copper-zinc system, alloys have the following melting end temperatures (liquidus temperatures) and boiling point temperatures:
The smallest difference between the temperatures of the end of melting and the beginning of boiling have alloys containing 40-46% Zn. This difference does not exceed 120 °C. Consequently, when overheated by only 120-130 ° C, these alloys begin to boil if melting is carried out at a pressure of 10–5 Pa. The introduction of zinc into liquid copper is always accompanied by boiling up of this metal, since tboil Zn = 905 ° C, and liquid copper is kept at 1150-1200 C during melting. The same phenomenon occurs when magnesium is introduced into liquid copper and its alloys (tboil = 1100 ° C), cadmium = 760 °C), phosphorus (tboil = 280 °C). The introduction of magnesium into molten cast iron (for the purpose of modifying and obtaining a spherical shape of graphite) is accompanied by a rapid boiling process of this metal, since the melt has a temperature of at least 1300 °C, and magnesium, having tboil = 1100 °C, is practically insoluble in this melt.
SECOND STAGE OF STEEL PRODUCTION - BOILING
STEEL PRODUCTION
A new feat has been achieved by adding surfactants to water - essentially creating a soapy liquid. Surfactant molecules that carry an electrical charge can be attracted or repelled by a metal surface by reversing the polarity of the voltage applied to the metal. Wang explains that this turns the metal surface between hydrophilic and hydrophobic.
Purpose of alloy steel
The addition of a surfactant causes the surface to become more hydrophobic, which increases the rate of nucleation to form bubbles. But a change in the charge on the surface causes the surface to become hydrophilic and prevents the formation of bubbles. The researchers found that they could achieve a tenfold change in the rate of bubble formation by simply switching the charge.
Steel- This is an iron-carbon alloy that contains about 1.5% carbon, if its content increases, then the brittleness and hardness of the steel increases significantly. The main source material for steel production- steel scrap and pig iron.
First of all, iron is oxidized during the interaction of oxygen and cast iron in steel furnaces. Together with iron, phosphorus, silicon, carbon and manganese are oxidized. Iron oxide, which is formed at high temperature regime, gives its oxygen in cast iron to more active impurities, while oxidizing them.
Just as condensation, such as the formation of raindrops, requires "seeds" like a dust particle to begin the nucleation process, bubbles formed by boiling water also require nucleation. Tiny bumps on the metal surface can provide these nucleation points, but if the surface is hydrophilic, bubble formation is inhibited.
“The whole concept is based on whether a hydrophobic or hydrophilic surface affects the nucleation rate,” says Cho. "If it's hydrophilic, it's very difficult to nucleate bubbles." Thus, by switching the polarity, the bubbling speed can be precisely controlled.
Steel production is carried out in three stages.
FIRST STAGE OF STEEL PRODUCTION - ROCK MELTING
The charge is melted and the bath of liquid metal is heated. The temperature of the metal is low, iron is vigorously oxidized, iron oxide is formed and impurities are oxidized: manganese, silicon and phosphorus.
The most important task of this stage steel production is the removal of phosphorus. To do this, it is necessary to carry out melting in the main furnace, where the slag will contain calcium oxide (CaO). Phosphoric anhydride - P2O5 will form a weak compound (FeO) 3 x P2O5 with iron oxide. Calcium oxide - as a stronger base than iron oxide, and at not very high temperatures binds P2O5 and turns it into slag.
Unlike other approaches to modifying the wettability of metal surfaces based on creating precise views of nanoscale textures on the surface, this system exploits the tiny roughness that naturally exists on the metal surface and does not require special processing.
The ability to actively control the rate of bubble formation, in turn, allows you to control the rate of heat transfer between the metal and the liquid. This would make it possible to make more efficient boilers for power plants or other applications, since for modern designs a significant margin of safety is required to avoid the possibility of hot spots that can seriously damage the equipment. While most such power plants are stable most of the time, the ability to dynamically adjust heat transfer rates can improve their efficiency as they rise or fall from full power and thus make it easier to make real-time changes to their output without loss of efficiency.
In order to remove phosphorus, a not very high temperature, slag and metal baths, and a sufficient content of FeO in the slag are needed. In order to increase the content of FeO in the slag and accelerate the oxidation of impurities, scale and iron ore are added to the furnace, inducing iron slag. Gradually, as phosphorus is removed from the metal into the slag, the content of phosphorus in the slag increases. So you need to remove this slag from the metal mirror, and then replace it with a new one with fresh additions of calcium oxide.
Likewise, liquid cooling for high-end electronics could also be more efficient by being able to control the bubbling speed to prevent overheating at hot spots, the team says. This system, Cho adds, provides "the ability to select the best heat transfer profile as needed," rather than choosing a uniform nucleation behavior that allows the fields to be used for the most extreme heat that this device has ever been expected to. “This allows you to select the optimum heat transfer rate instantly,” he says. “By having a boiler that can respond to rapid changes,” it can provide additional flexibility for the electrical grid. "It gives you an extra handle" to control the system.
SECOND STAGE OF STEEL PRODUCTION - BOILING
The metal bath is boiling. It begins gradually, as it warms up to high temperatures. With an increase in temperature, the oxidation reaction of carbon proceeds more intensively, proceeding with the absorption of heat.
In order to oxidize carbon, a small amount of scale, ore is introduced into the metal, or oxygen is blown in. When carbon reacts with iron oxide, bubbles of carbon monoxide are removed from the liquid metal, and "boiling the bath" occurs. During the “boiling”, the carbon content in the metal is reduced to the required amount, the temperature is equalized over the volume of the bath, non-metallic inclusions that stick to the rising CO bubbles and gases that penetrate the CO bubbles are slightly removed. All this leads to an increase in the quality of the metal. This means that this stage is the main one in the steel production process.
Wang says that this work has demonstrated that you can actively change the nucleation rate. This has not previously been shown to be possible. Power plant operators are rightly conservative about making changes, Cho says, because people depend on their products, so while only relatively minor changes are needed for this system, a demonstration plant will be required to prove the concept at operational scale. But "I don't think there are any huge barriers" to such a demonstration, he says. “Theoretically, it should be easy,” Wang says, although only with a full-scale system can it be shown that the benefits outweigh the installation costs.










The legacy of the Atlantean civilization
What is the dream of the red stone
Dream interpretation of the royal family. The king dreamed. Old Russian dream book
What to do to increase hemoglobin during pregnancy: products, pills, general recommendations Products to increase hemoglobin in pregnant women
The concept of negativism: symptoms and features of manifestation in children and adults